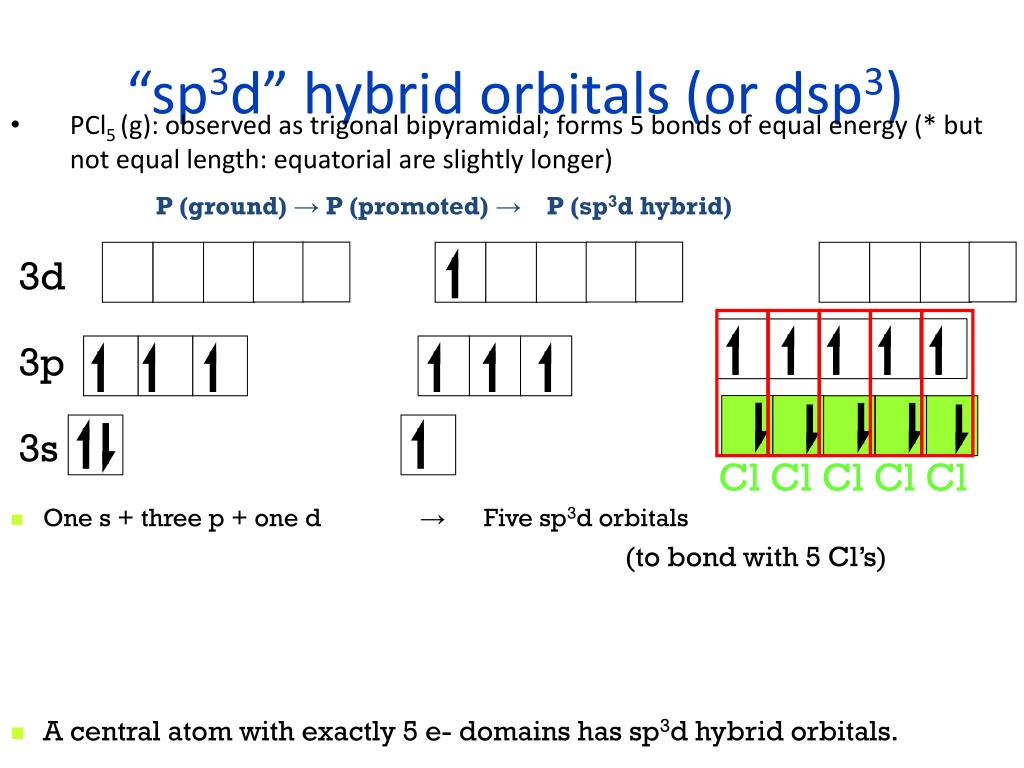
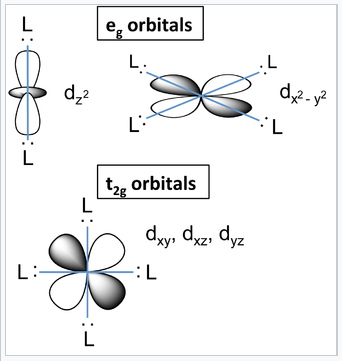
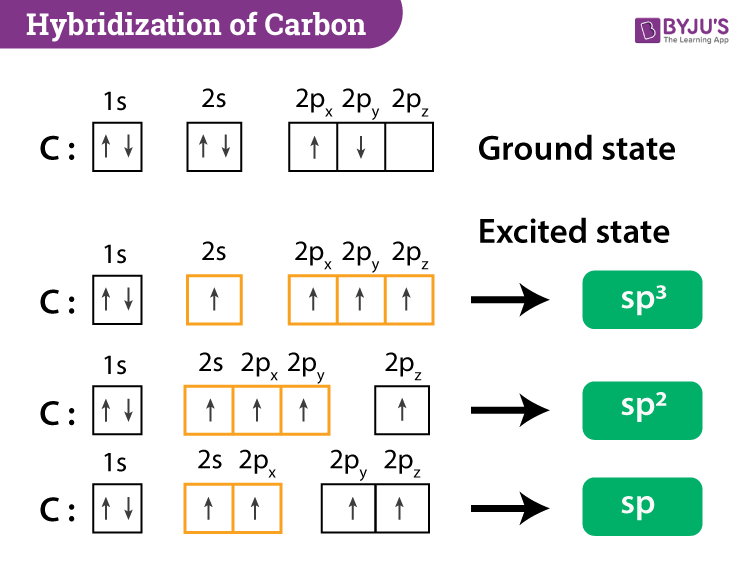
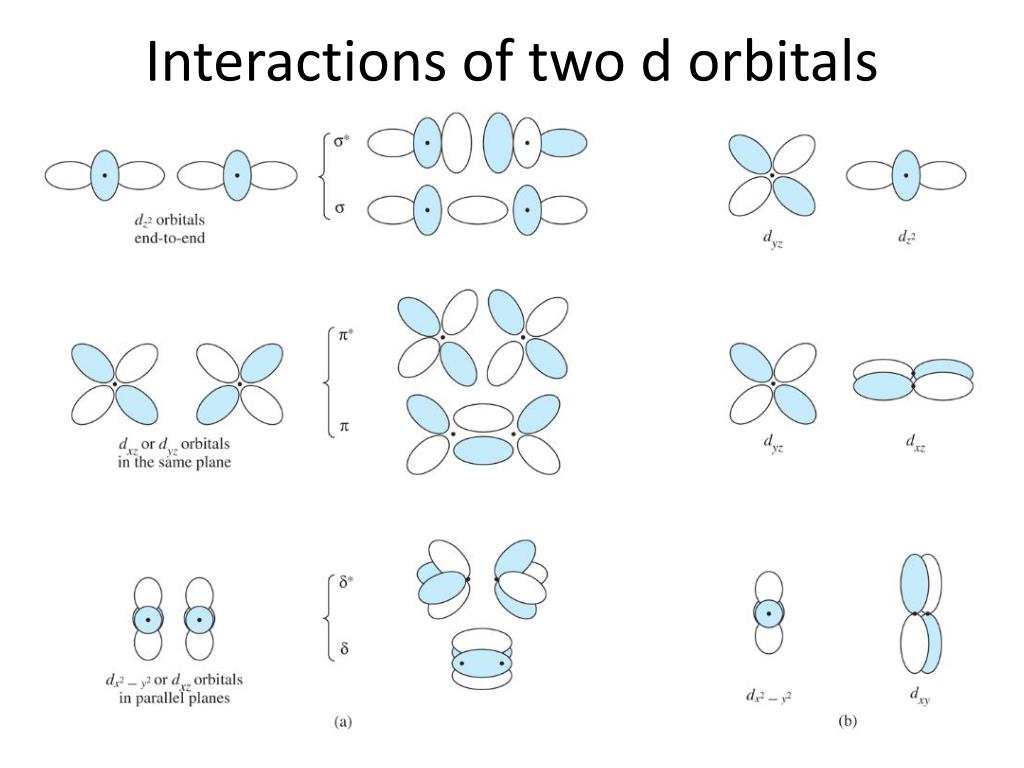
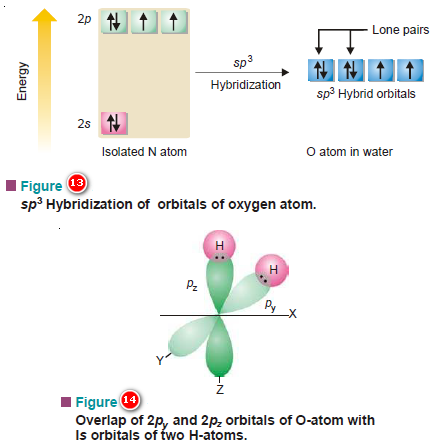
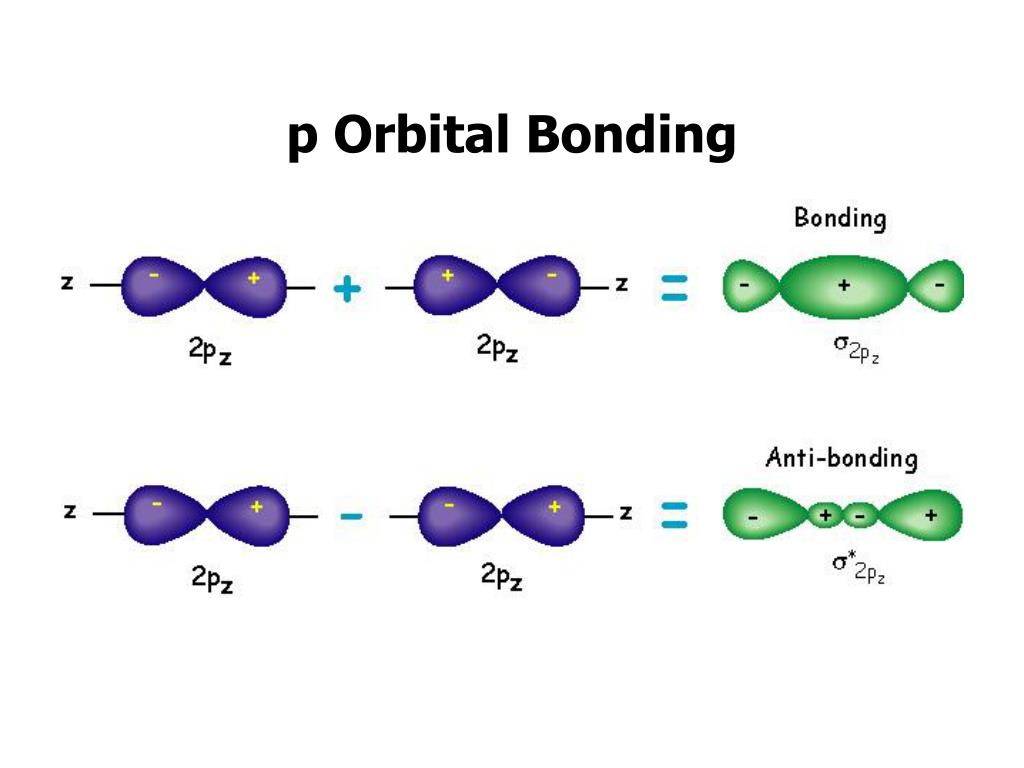
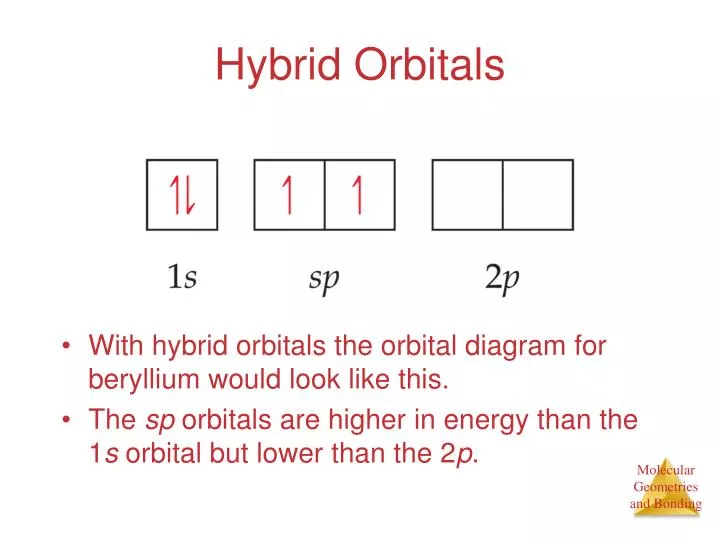
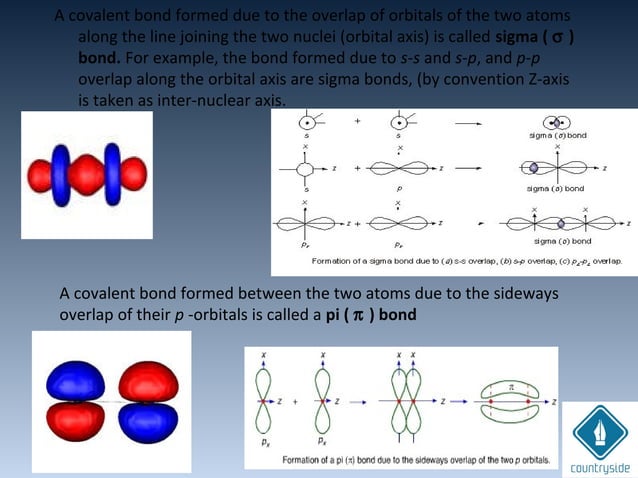
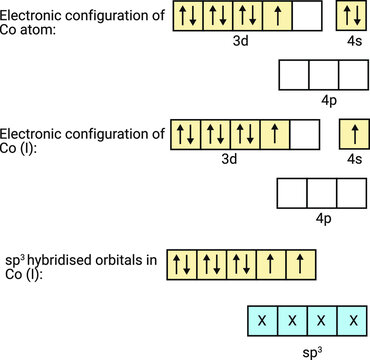
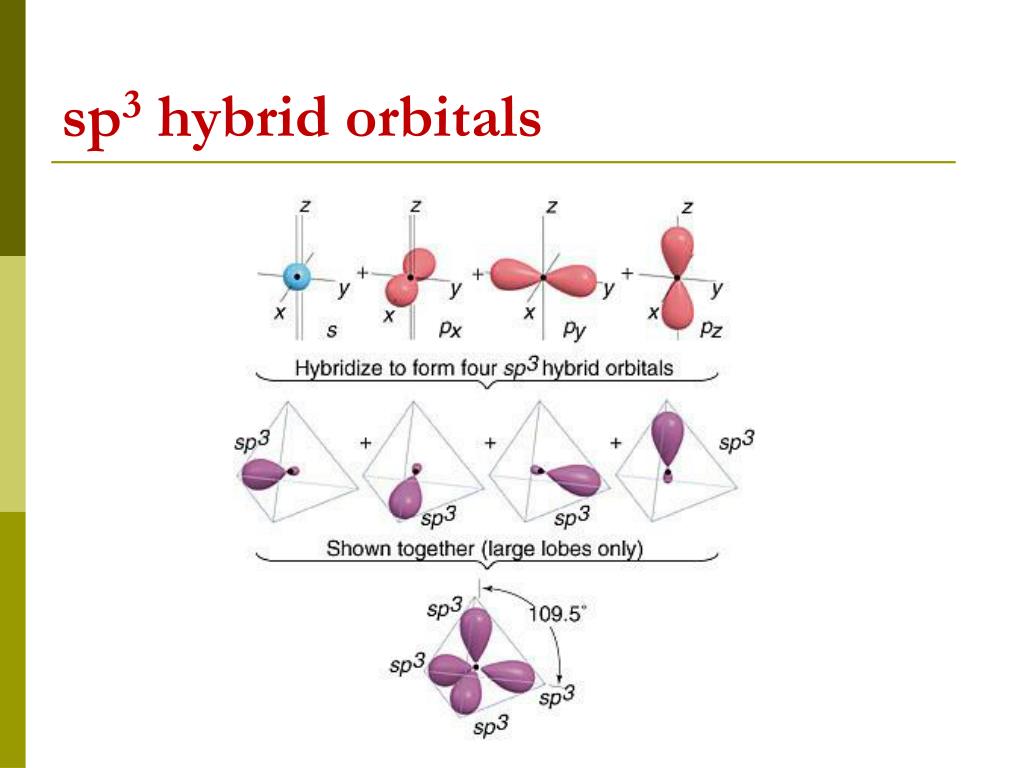
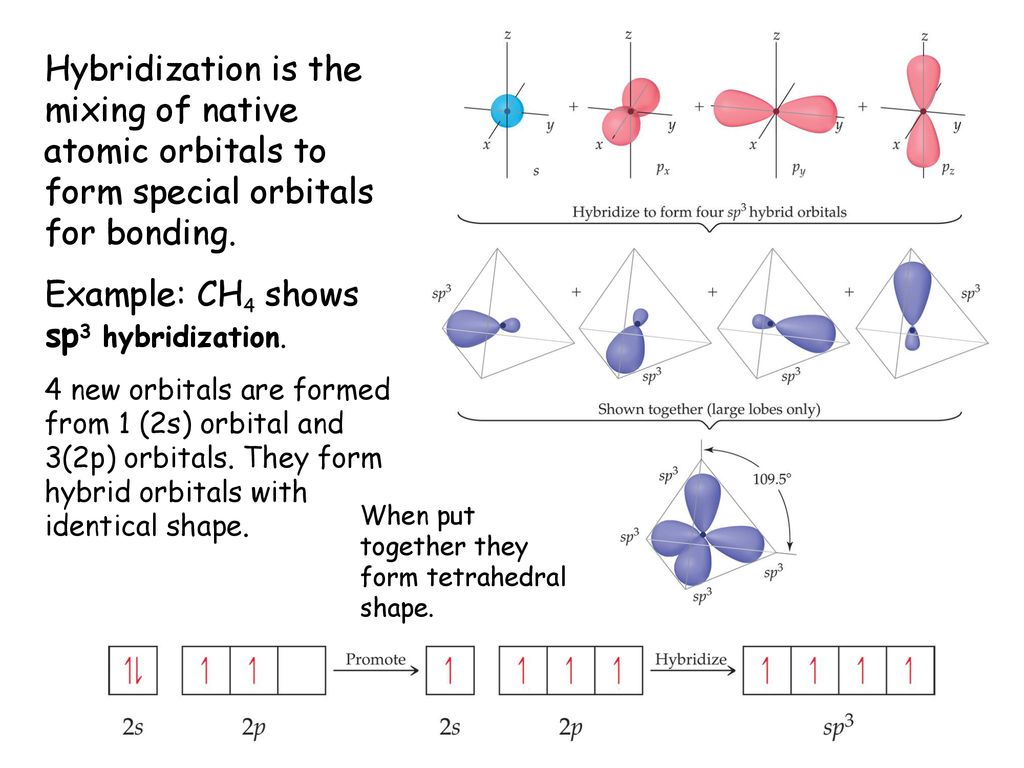
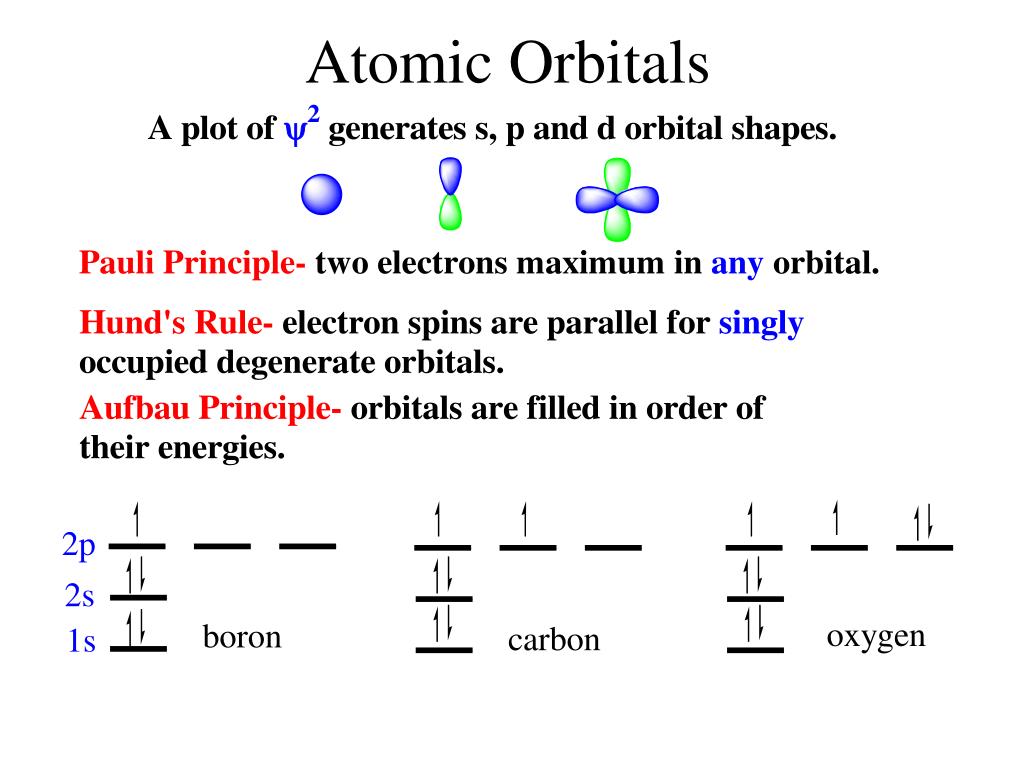
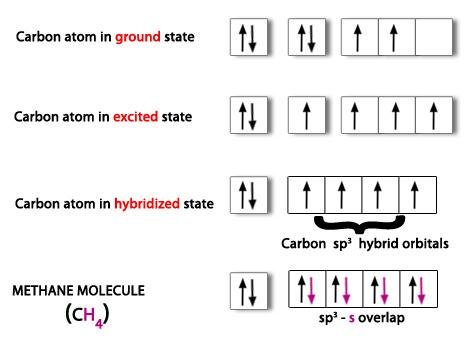
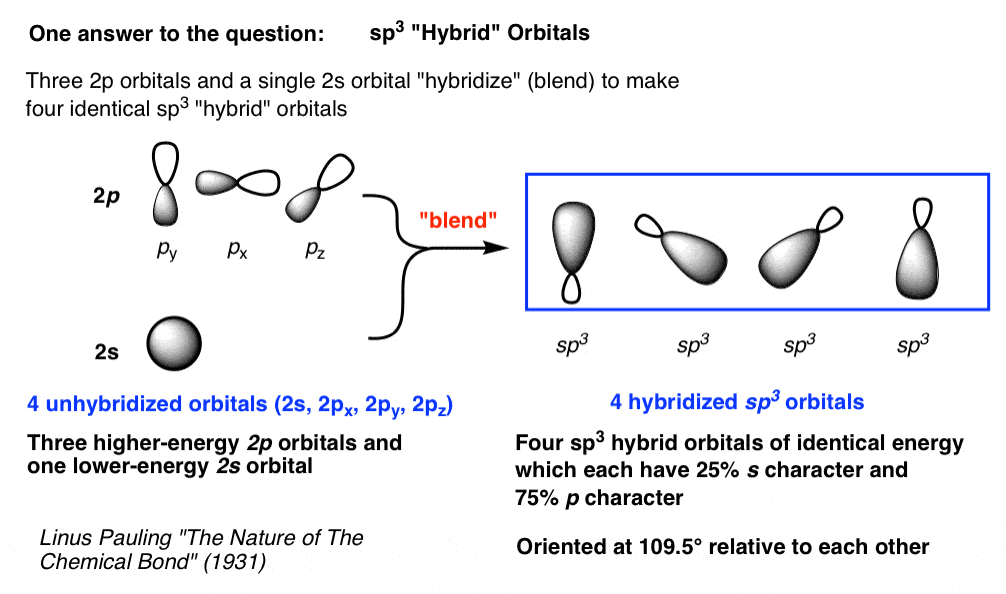
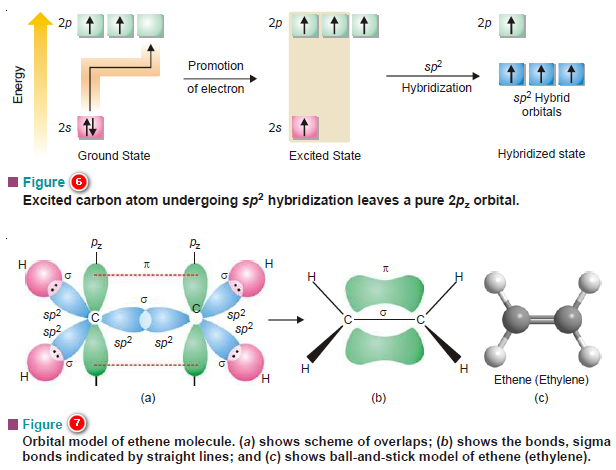
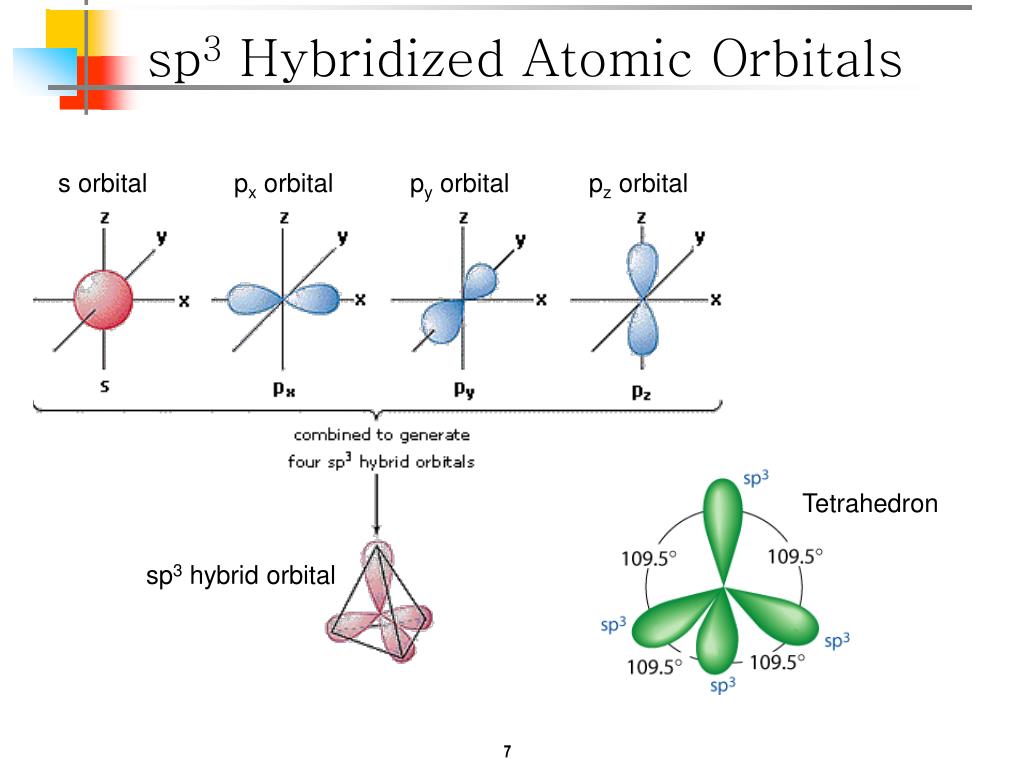
PPT - VSEPR Theory PowerPoint Presentation - ID:3260916
Lone pairs repel more strongly than bonding pairs!!!. VSEPR Theory. Types of e - Pairs Bonding pairs - form bonds Lone pairs - nonbonding electrons. Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem. Bond Angle. VSEPR Theory.
Lone pairs repel more strongly than bonding pairs!!! VSEPR Theory • Types of e- Pairs • Bonding pairs - form bonds • Lone pairs - nonbonding electron