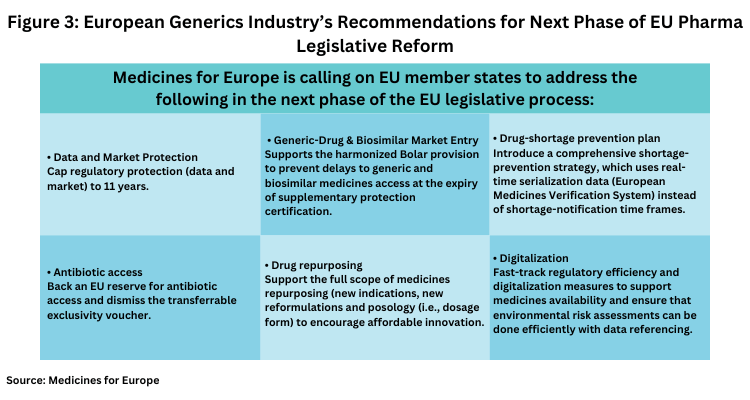
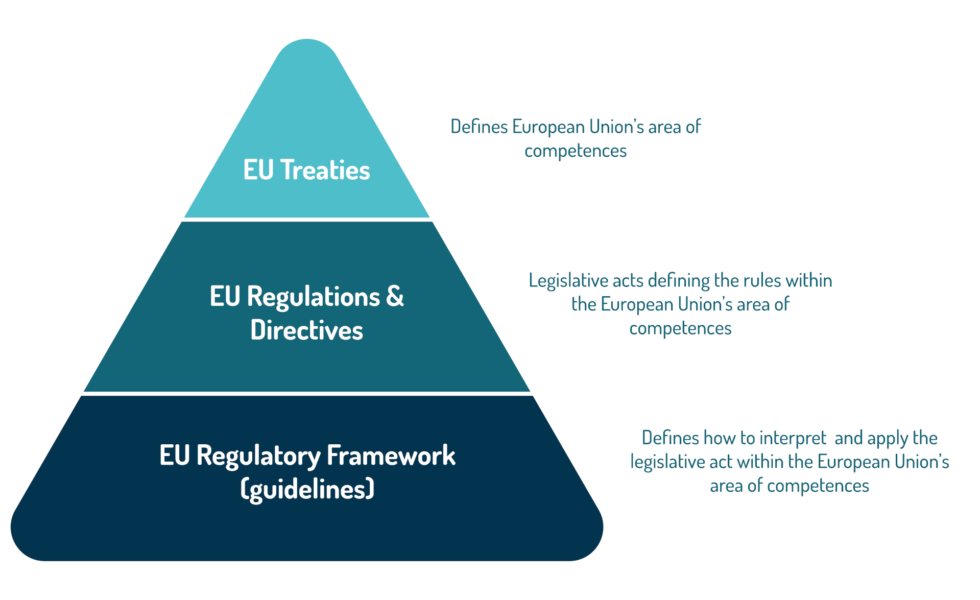
Utilize our extensive eu pharmaceutical legislation revisions: implications for biopharma resource library containing extensive collections of high-quality images. optimized for both digital and print applications across multiple platforms. providing reliable visual resources for business and academic use. Our eu pharmaceutical legislation revisions: implications for biopharma collection features high-quality images with excellent detail and clarity. Perfect for marketing materials, corporate presentations, advertising campaigns, and professional publications All eu pharmaceutical legislation revisions: implications for biopharma images are available in high resolution with professional-grade quality, optimized for both digital and print applications, and include comprehensive metadata for easy organization and usage. Professional photographers and designers trust our eu pharmaceutical legislation revisions: implications for biopharma images for their consistent quality and technical excellence. Professional licensing options accommodate both commercial and educational usage requirements. Multiple resolution options ensure optimal performance across different platforms and applications. Reliable customer support ensures smooth experience throughout the eu pharmaceutical legislation revisions: implications for biopharma selection process. Each image in our eu pharmaceutical legislation revisions: implications for biopharma gallery undergoes rigorous quality assessment before inclusion. Comprehensive tagging systems facilitate quick discovery of relevant eu pharmaceutical legislation revisions: implications for biopharma content. The eu pharmaceutical legislation revisions: implications for biopharma archive serves professionals, educators, and creatives across diverse industries.